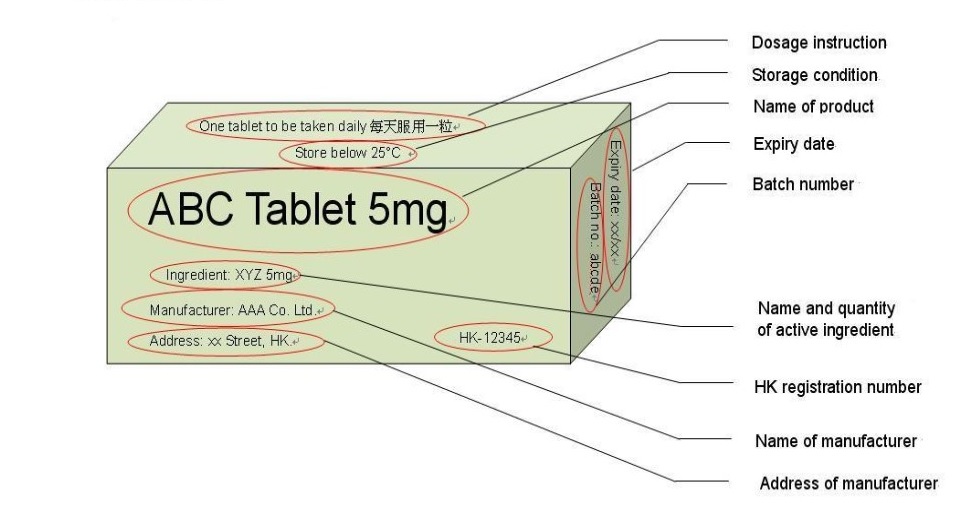
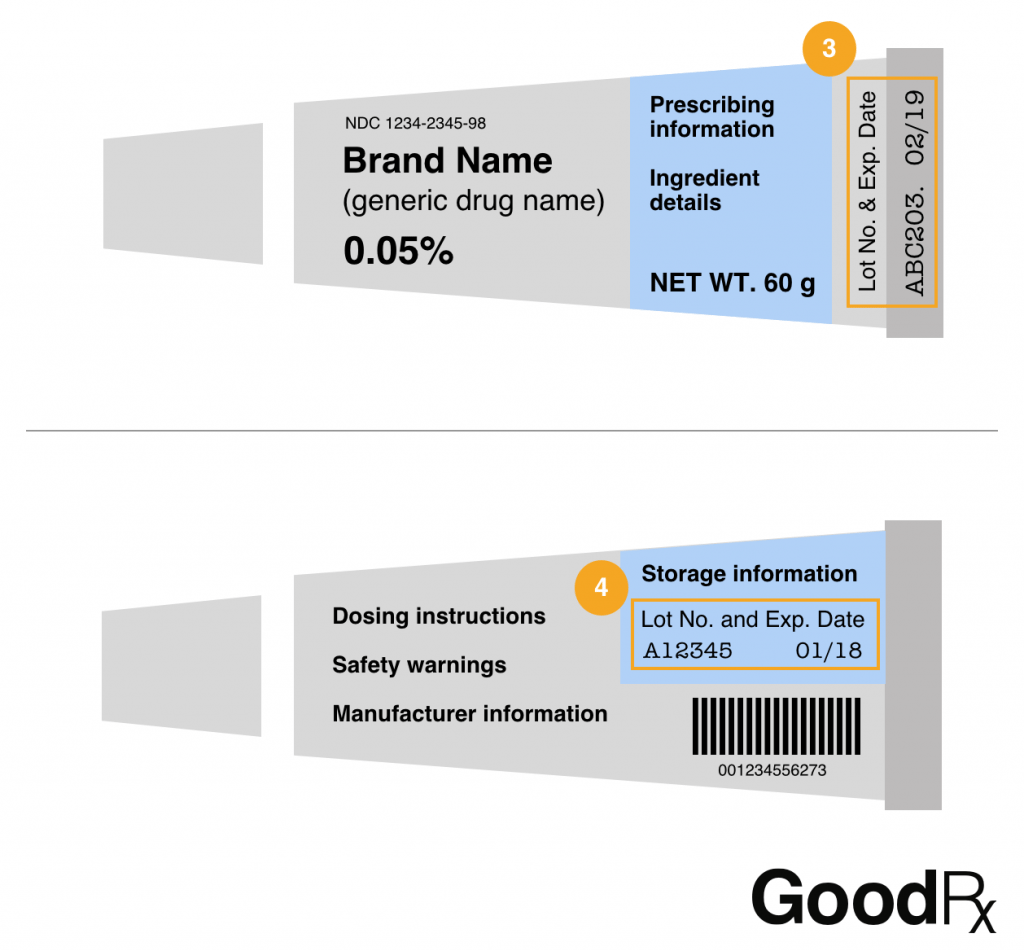
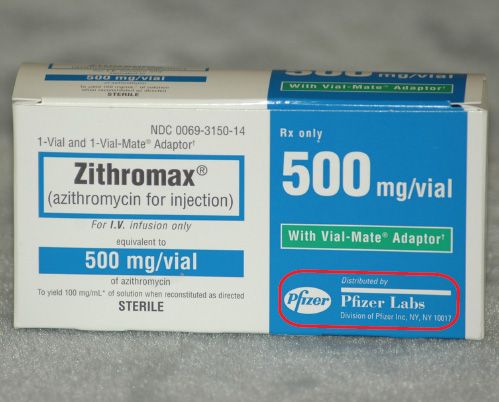
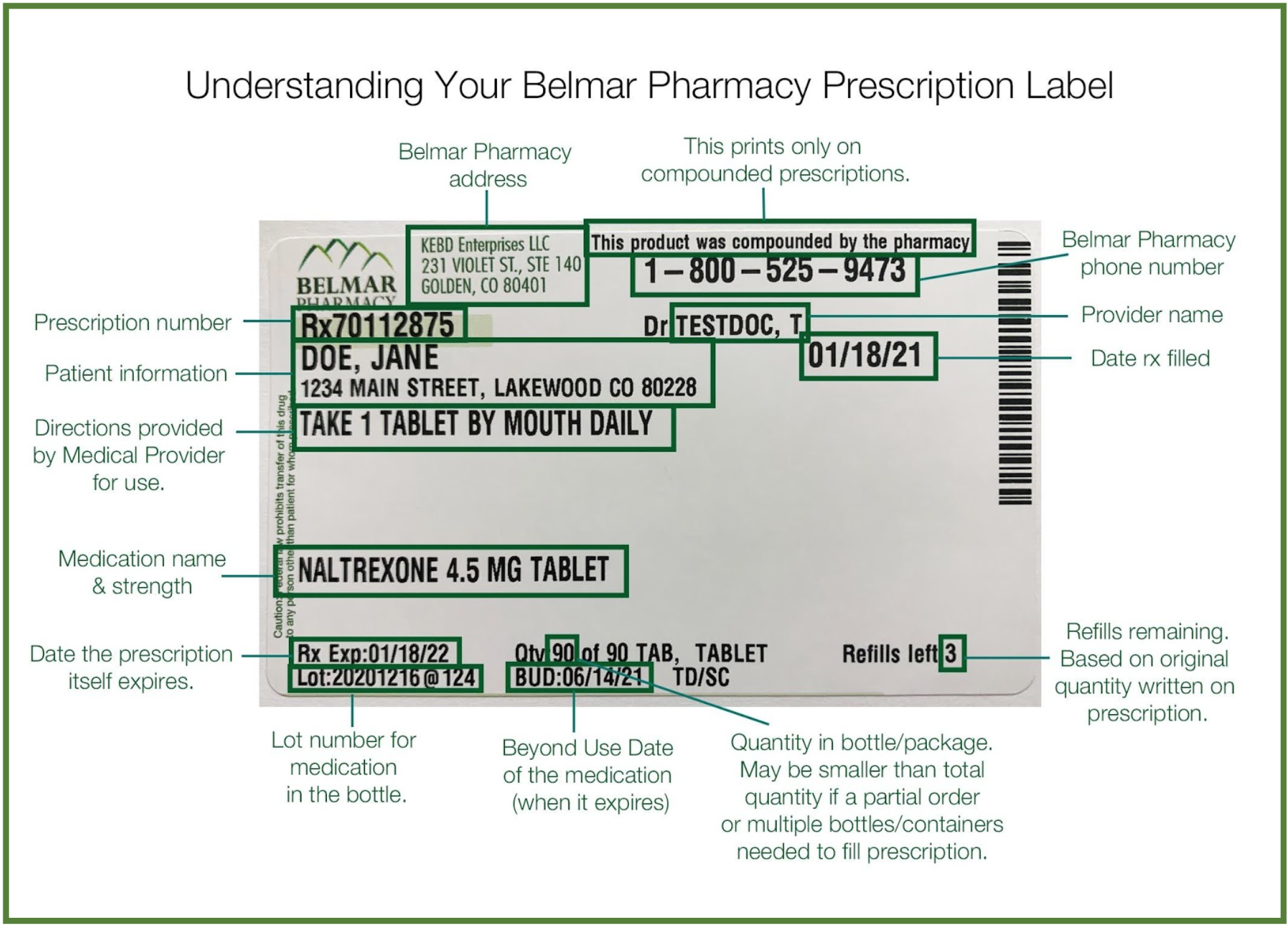
38 lot number on medication label
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 211.125 Labeling issuance. (a) Strict control shall be exercised over labeling issued for use in drug product labeling operations. (b) Labeling materials issued for a batch shall be carefully examined for identity and conformity to the labeling specified in the master or batch production records. (c) Procedures shall be used to reconcile ... Federal Register :: Bar Code Label Requirement for Human Drug Products ... One comment suggested amending § 201.25(c), regarding the bar code's placement on a label, to state that any drug complying with the bar code requirement is exempt from § 201.10(i)(1)(iii) and (i)(1)(iv) (provisions regarding the identifying lot number or control number and manufacturer's, packer's, labeler's, or distributor's name) if the ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The lot number on the label of a drug should be capable of yielding the complete manufacturing history of the package. An incorrect lot number may be regarded as causing the article to be misbranded. Sec. 201.19 Drugs; use of term "infant". The regulations affecting special dietary foods (§ 105.3(e) of this chapter) define an infant as a child ...
Lot number on medication label
How to Understand FDA Lot Numbers | Healthfully The lot number may or may not be labeled as such; in other words, you may see "lot" followed by the number, but not always. When looking for the lot number, keep in mind that it will not be printed as a standard part of the product label since the product label doesn't change, whereas the lot number changes with every batch. › Health_News › 2021/11/22Many psychiatric patients getting risky drug gabapentin ... - UPI Nov 22, 2021 · The new study found that of 130 million outpatient gabapentin prescriptions, more than 99% were for off-label uses. In the U.S., the drug is officially approved for treating certain seizures and ... The Over-the-Counter Medicine Label: Take a Look | FDA The Label Also Tells You... The expiration date, when applicable (date after which you should not use the product). Lot or batch code (manufacturer information to help identify the product). Name...
Lot number on medication label. templatelab.com › medication-schedule40 Great Medication Schedule Templates (+Medication Calendars) The name of each medication; The dosage of each medication; What each of the medicines does You can find this information on the bottle label or package insert; Suggestions about how you will depict this information through pictures; The table will now serve as your guide in creating an outline of the data that you will include on your medicine ... Lot number | definition of lot number by Medical dictionary lot number An identifier assigned to a batch of medications. It facilitates drug manufacturing inventory control and tracing adverse incidents in a batch of contaminated medications. See also: number Medical Dictionary, © 2009 Farlex and Partners Want to thank TFD for its existence? What is a lot number and how do I identify it? - Acme United ... The lot number identifies when a product was manufactured. It will be in small type, all capital letters, and say "LOT #". On many products, it's on the back panel. On kit boxes, it's either on the top or bottom of the box, generally affixed to a label. Lot numbers have 2 parts: a letter, followed by a series of numbers. 21 CFR § 203.38 - Sample lot or control numbers; labeling of sample ... The manufacturer or authorized distributor of record of a drug sample shall include on the label of the sample unit and on the outside container or packaging of the sample unit, if any, an identifying lot or control number that will permit the tracking of the distribution of each drug sample unit.
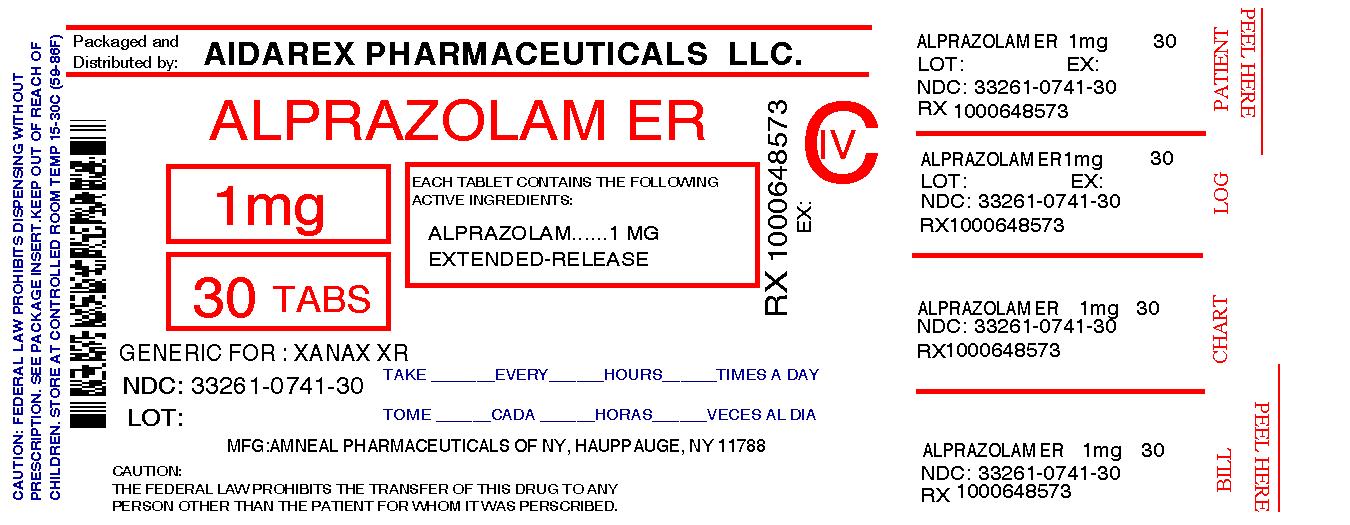
mattlaw.com › pharmacy-error-wrong-medicationPharmacy Error (When Pharmacist Gives Wrong Medication) she became severly tired sleeping almost 10 to 12 hors a day waking up in time to take her medication. upon checking her medication , the tablet was pink with the number 262 on it. the medication pantoprazole was for a stomach bacteria infection. the pink pill is used to treat schizophrenia. do she have any legal recourse. reply Lot Pill Images - Pill Identifier - Drugs.com Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. This material is provided for educational purposes only and is not intended for medical advice, diagnosis or treatment. Data sources include IBM Watson Micromedex (updated 1 Sep 2022), Cerner Multum™ (updated 5 Sep 2022), ASHP (updated 1 July 2022 ... file.cop.ufl.edu › ce › consultwbCHAPTER 20 LABELING MEDICATIONS AND EXPIRATION DATING A. UNIT DOSE MEDICATION – (Prepackaging) reference 64F-12.006 Minimum labeling to include: a) Name of drug (brand or generic or both) b) Strength c) Dosage Form d) Manufacturer e) Lot number f) Expiration date/beyond use date g) OR instead of (d) and (e) a control number which cross references to the manufacturer name and lot number › news › press-releasePfizer Voluntary Nationwide Recall of Lots of ACCURETIC ... Mar 21, 2022 · The NDC, Lot Number, Expiration Date, and Configuration details for these products are indicated in the tables below and photos of the products can be found at the end of this press release. The product lots were distributed nationwide to wholesalers and Distributors in the United States and Puerto Rico from November 2019 to March 2022.
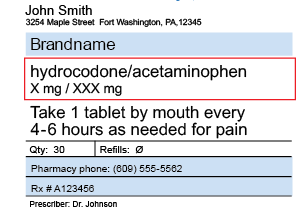
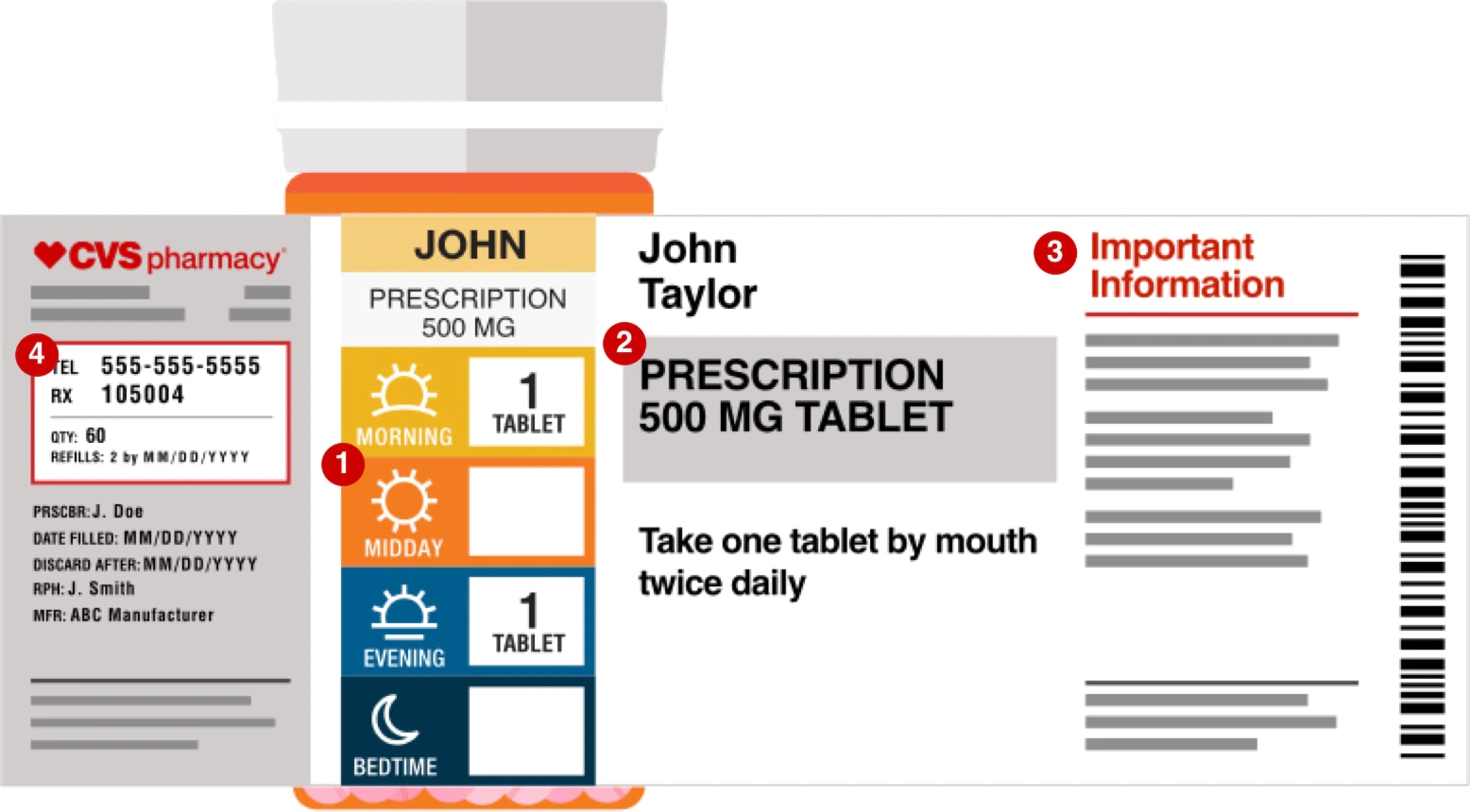
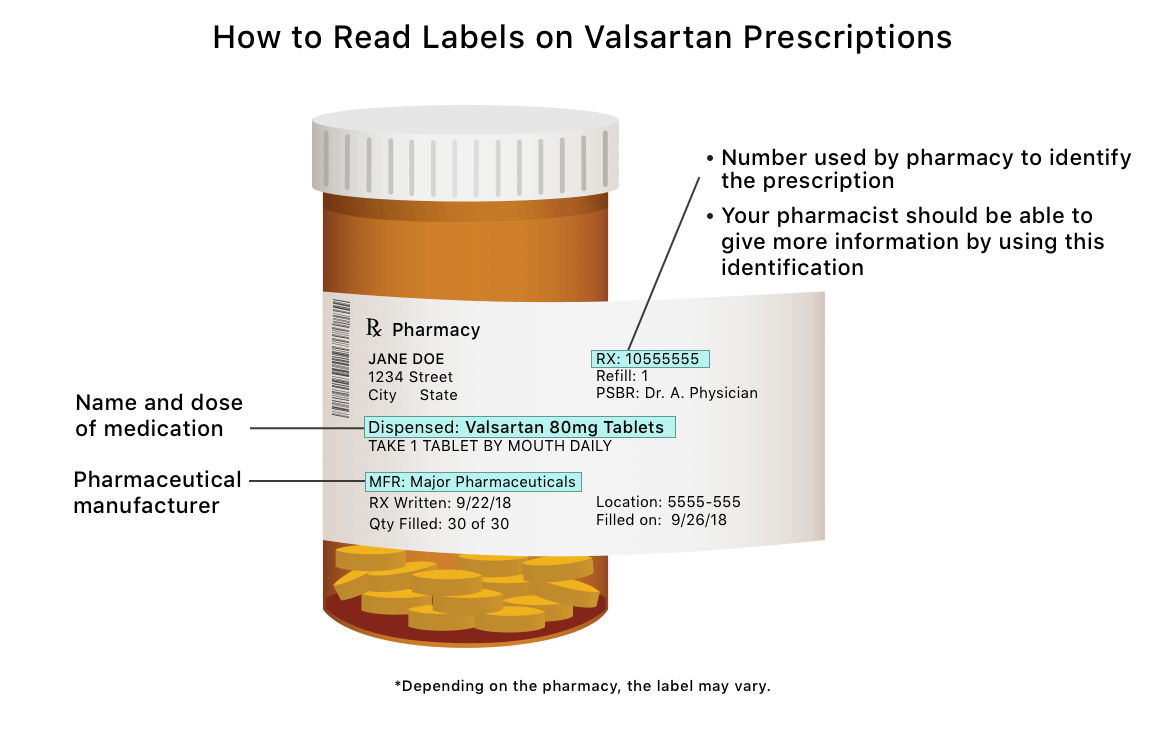
Packaging and Labeling - Food and Drug Administration Division of Drug Quality, Office of Manufacturing Quality ... are ³cut´ from a sheet or roll of labels b) Cut labeling operations shall include one of the following: ... ¨ Lot or control number ... CVS pharmacy - Frequently Asked Questions Your label has the following information: Your name Your prescription name and number Directions for use The quantity of the medication The number of refills and prescription expiration date The phone number and store number of the CVS/pharmacy where you filled the prescription Your prescriber's name Safety Considerations for Container Labels and Carton Labeling to ... -Lot number -Name of manufacturer, packer, or distributor • USP requires labels of official drug product to bear an expiration date • Biologic Products: Minimum requirements under 21 CFR 610.60(c)... Amazon.com: Medication Labels - Recordkeeping & Labels: Industrial ... 1-24 of 214 results for "Medication Labels" RESULTS PDC Healthcare 59704609 Paper Label, Permanent, Medication Added, 3" x 2", Red (Pack of 500) 9 $2023 ($0.04/Count) Save more with Subscribe & Save Get it as soon as Fri, Sep 2 FREE Shipping on orders over $25 shipped by Amazon
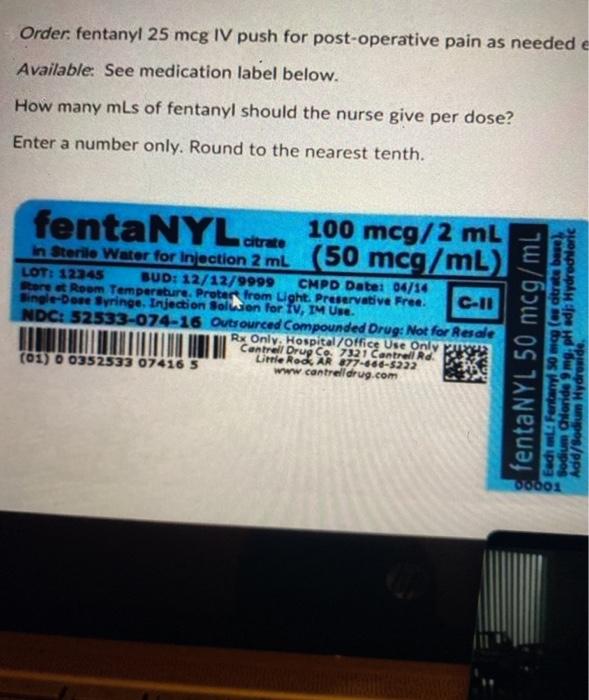
Federal Register :: Potential Medication Error Risks With ... While not a regulatory requirement, some investigational new drug container labels may include additional information such as the protocol/clinical trial number, concentration and/or strength, dosage form ( e.g., tablets, injection), quantity per container, storage requirements, and lot number.
FDA Label Search - Food and Drug Administration The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) The drug labeling and other information has been reformatted to make it easier to read but its content has neither been altered nor ...
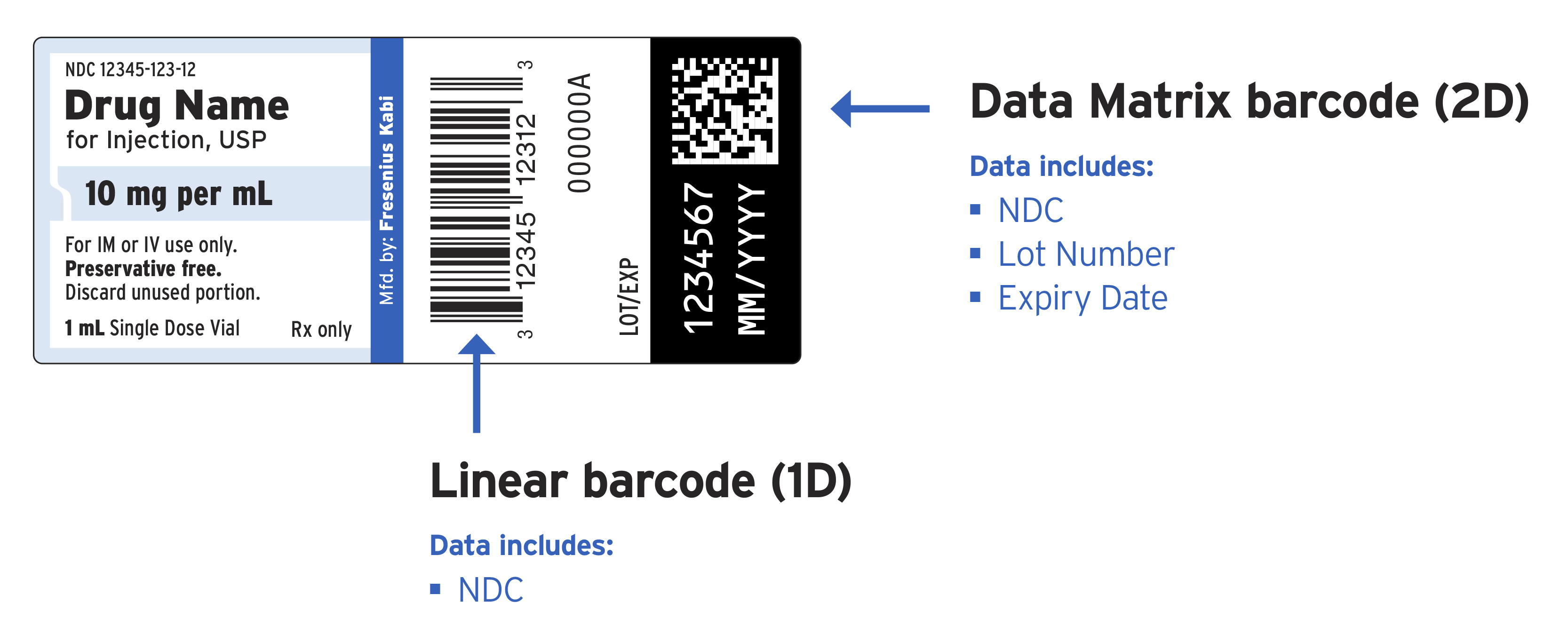
Quality System Regulation Labeling Requirements | FDA Most labeling, however, also contains another number, such as a drawing number, for control of labeling configuration and procurement. The control number for traceability need not be on every label...
Guidance Document: Labelling of Pharmaceutical Drugs for Human Use Labelling of Pharmaceutical Drugs for Human Use replaces the Health Canada guidance document Labelling of Drugs for Human Use. This guidance document came into effect in 1989, was subsequently revised in 1991, and has since been removed from circulation by Health Canada because much of its content was deemed to be out-of-date.
› transportationTransportation - New Albany Floyd County Administration Business Services Employee Contracts Facilities Food & Nutrition Services Human Resources Office of the Superintendent Teaching & Learning Title IX Regulations Technology Resources Transportation Get involved Explore Our Schools Please read all directions on this page before clicking on the bus routing link below: You may access bus stop information for your student by
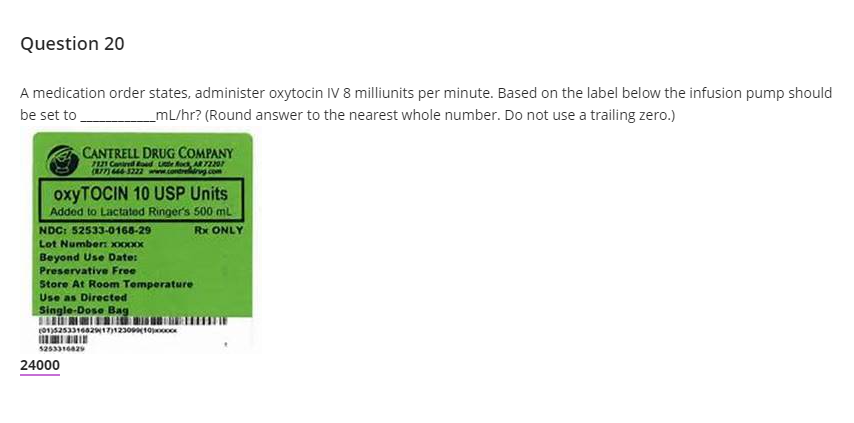
Drug Recalls: What to Do and How to Find Your Medication Lot Number ... 2) Find your medication's lot number. Drug recalls pertain to certain lots of the medication that were made during a given time period. To find out which lot numbers were affected by a recall, read the official recall announcement either on the manufacturer's website or on the FDA's website here.
› scripts › cdrhCFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (a) Lot or control number required on drug sample labeling and sample unit label. The manufacturer or authorized distributor of record of a drug sample shall include on the label of the sample unit and on the outside container or packaging of the sample unit, if any, an identifying lot or control number that will permit the tracking of the ...
The Over-the-Counter Medicine Label: Take a Look | FDA The Label Also Tells You... The expiration date, when applicable (date after which you should not use the product). Lot or batch code (manufacturer information to help identify the product). Name...
› Health_News › 2021/11/22Many psychiatric patients getting risky drug gabapentin ... - UPI Nov 22, 2021 · The new study found that of 130 million outpatient gabapentin prescriptions, more than 99% were for off-label uses. In the U.S., the drug is officially approved for treating certain seizures and ...
How to Understand FDA Lot Numbers | Healthfully The lot number may or may not be labeled as such; in other words, you may see "lot" followed by the number, but not always. When looking for the lot number, keep in mind that it will not be printed as a standard part of the product label since the product label doesn't change, whereas the lot number changes with every batch.





















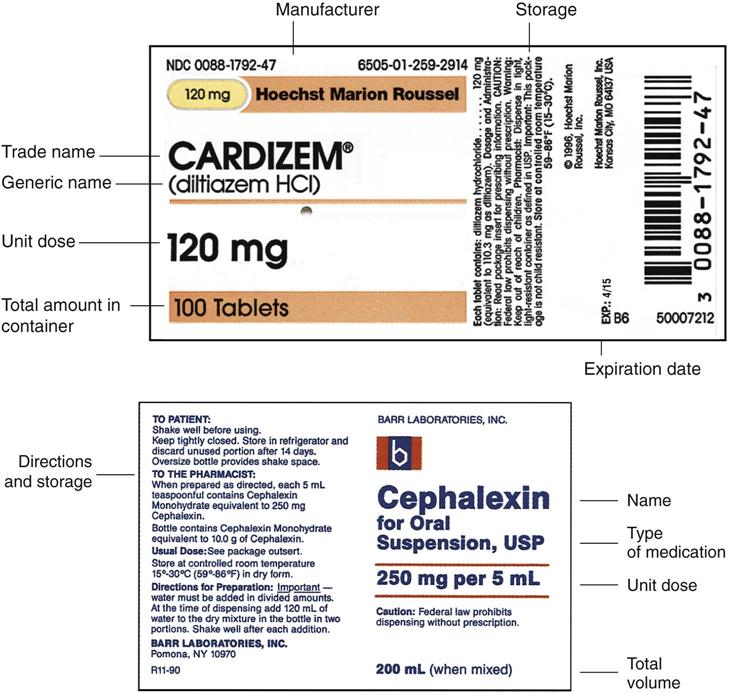
Post a Comment for "38 lot number on medication label"